How Air Pollution Accelerates Corrosion & How to Stop It
Corrosion is often overlooked in indoor air quality (IAQ) discussions, yet its global economic impact is staggering—estimated at over 3% of global GDP annually, according to NACE (National Association of Corrosion Engineers). While most IAQ efforts focus on health and particulate control, airborne contaminants like acidic gases, sulfur compounds, and fine metal particulates quietly degrade vital infrastructure.
From premature failure of HVAC systems and electronic equipment in data centers, to accelerated deterioration of bridges and reinforced concrete exposed to polluted air, corrosion leads to substantial unplanned costs. It also threatens cultural heritage sites, where even trace levels of corrosive gases can irreversibly damage stone, metals, and historic interiors.
Despite its consequences, corrosion is rarely addressed in IAQ strategies—largely because it’s not visible until damage is done. Monitoring for corrosive air pollutants, deploying corrosion-grade air filters, and implementing controlled pressurization and filtration systems are critical steps toward mitigating this often-hidden risk.
The cost of ignoring corrosion isn’t just economic—it’s structural, cultural, and operational.
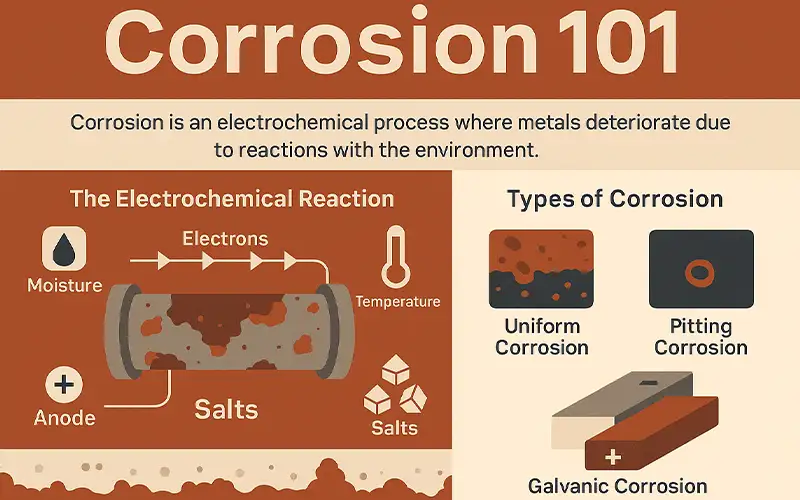
Corrosion 101
Corrosion is a natural electrochemical process in which metals deteriorate due to reactions with their environment. At its core, corrosion involves the transfer of electrons between metal surfaces and external elements such as oxygen, water, or salts. This reaction forms metal oxides or other compounds, weakening structural integrity over time.
The Electrochemical Reaction Behind Corrosion
When a metal is exposed to moisture and oxygen, it becomes an anode in an electrochemical cell. Electrons flow from the anodic region to a cathodic region, often in the presence of electrolytes like salt or acids. This process leads to oxidation and the formation of rust or other corrosion products.
Environmental Factors
Several environmental conditions accelerate corrosion:
Moisture: Water acts as a conductor, enabling electron movement.
Temperature: Higher temperatures typically increase corrosion rates.
Salts: Common in coastal and industrial areas, salts enhance conductivity and lower the pH of surface moisture, speeding up metal degradation.
For more on the chemistry of corrosion, visit Corrosion Doctors.
Types of Corrosion
Uniform Corrosion: Occurs evenly across a surface. It is predictable and easier to monitor and mitigate.
Pitting Corrosion: Involves localized attacks that form deep pits or holes. This type can lead to sudden failures, especially in stainless steel and aluminum components.
Galvanic Corrosion: Happens when two dissimilar metals are electrically connected in a conductive environment. The less noble metal corrodes faster, while the more noble metal remains protected.
Understanding the types and triggers of corrosion is essential in designing air filtration systems for industrial plants, data centers, and heritage sites. To explore classification standards and prevention methods, refer to NACE International.
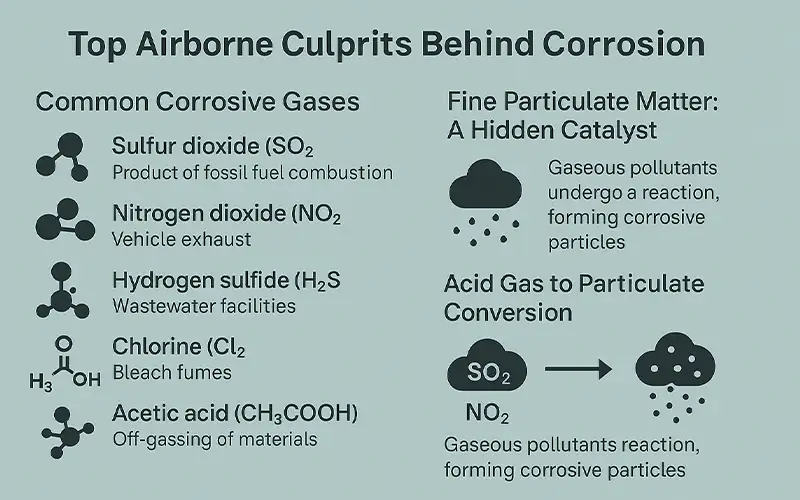
Top Airborne Culprits Behind Corrosion
Several airborne pollutants are known to accelerate corrosion, particularly in industrial, coastal, and urban environments. These corrosive agents exist in gaseous and particulate forms, and their interaction with indoor surfaces can result in long-term structural damage and electronic failures.
Common Corrosive Gases
Sulfur dioxide (SO₂): A major byproduct of fossil fuel combustion, SO₂ reacts with moisture to form sulfurous and sulfuric acid, corroding metals, stone, and electronics.
Nitrogen dioxide (NO₂): Found in vehicle exhaust and combustion processes, NO₂ can generate nitric acid in humid environments, promoting rust and degradation of finishes.
Hydrogen sulfide (H₂S): Common in wastewater facilities and pulp mills, H₂S corrodes copper, silver, and brass, and causes tarnishing of sensitive electronic components.
Chlorine (Cl₂): Present in bleach fumes, coastal air, and industrial facilities, chlorine contributes to stress corrosion cracking and rapid pitting.
Acetic acid (CH₃COOH): Off-gassed from wood products, adhesives, and museum displays, this organic acid is particularly corrosive to metals like copper and lead in archival and heritage spaces.
For more details on these gases and their corrosive impact, visit the U.S. Environmental Protection Agency (EPA).
Fine Particulate Matter: A Hidden Catalyst
Fine particulate matter (PM₂.₅ and smaller) may contain embedded sulfates, nitrates, heavy metals, and salt residues that accelerate surface corrosion. These particles settle on equipment, combine with ambient humidity, and form corrosive films.
Acid Gas to Particulate Conversion
Gaseous pollutants like SO₂ and NO₂ often undergo atmospheric reactions, especially in the presence of ammonia, forming sulfate and nitrate salts. These salts attach to dust particles, transforming acidic gases into fine PM that deposits on surfaces. The result is a sticky, corrosive layer that may go unnoticed until irreversible damage is done.
This conversion makes it essential to filter both gases and fine particles in sensitive environments such as data centers, cleanrooms, and heritage archives. Learn more about acid-gas chemistry in EPA’s Air Pollution Topics.
Real-World Damage Portfolio from Corrosive Air Pollutants
Corrosive airborne contaminants don’t just degrade aesthetics—they inflict measurable damage across industries and environments. From infrastructure failure to loss of cultural heritage, the effects of unchecked corrosive air are costly and often irreversible.
Infrastructure Deterioration
Airborne pollutants like sulfur dioxide (SO₂), nitrogen dioxide (NO₂), and chlorides can penetrate porous concrete and react with embedded steel reinforcement. This leads to rebar corrosion, cracking, and eventual structural failure. Electrical towers and bridges exposed to polluted, humid environments also suffer metal loss and fatigue, compromising safety and requiring expensive repair.
Manufacturing Downtime
In electronics, semiconductor, and precision manufacturing, airborne sulfur and chlorine compounds cause circuit corrosion, sensor failure, and increased maintenance cycles. Even trace amounts of these pollutants can lead to unscheduled downtime, yield loss, and higher equipment turnover—especially in data centers and cleanrooms.
Damage to Museum Artefacts
Museum collections and archival materials are extremely vulnerable to corrosive gases such as acetic acid and hydrogen sulfide. These compounds tarnish metals like silver and copper, fade textiles, and degrade organic materials such as parchment and wood. Without adequate air filtration, priceless artefacts suffer irreversible chemical damage over time.
Household and Office Electronics
In residential and office settings, corrosive particles and gases contribute to early failure of consumer electronics, including laptops, phones, HVAC controls, and televisions. Fine particulate matter and acid gases form films on circuit boards and solder joints, causing oxidation, short circuits, and permanent performance loss.
Recognizing these real-world consequences highlights the need for corrosion-targeted air filtration, especially in mixed-use or high-sensitivity environments. Preventative air quality control can reduce lifecycle costs, protect critical assets, and extend infrastructure longevity.

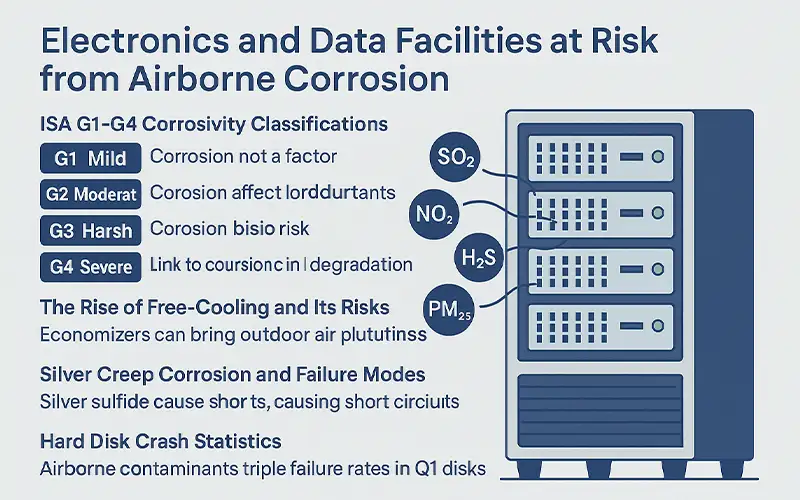
Electronics and Data Facilities at Risk from Airborne Corrosion
Modern electronics and data centers operate in highly sensitive environments where even low concentrations of corrosive gases and fine particulates can compromise reliability. As server density rises and energy-saving practices expand, the risk of airborne-induced failures grows—especially when filtration and humidity control are insufficient.
ISA G1–G4 Corrosivity Classifications
The International Society of Automation (ISA) has established a classification system for airborne corrosivity in electronic environments, known as ISA-71.04-2013:
G1 (Mild): Corrosion is not a factor for equipment reliability
G2 (Moderate): Corrosion may affect long-term reliability
G3 (Harsh): High risk of corrosion-related failure
G4 (Severe): Equipment degradation is expected within months
Many uncontrolled or semi-conditioned spaces, particularly those using outdoor air, exceed G2 or even G3 levels—leading to premature hardware failures and warranty claims.
The Rise of Free-Cooling and Its Risks
To save energy, many facilities have adopted free-cooling or economizer systems, which use outside air to cool servers rather than relying on chilled water or refrigerant-based systems.
While efficient, these systems introduce outdoor pollutants like SO₂, NO₂, H₂S, and chloride-laden PM into the server room, bypassing traditional mechanical filtration unless advanced air quality control is in place.
Without gas-phase filtration, these contaminants settle on circuit boards and metal surfaces, accelerating corrosion.
Silver Creep Corrosion and Failure Modes
One of the most common and insidious forms of airborne-induced failure is silver creep corrosion. Sulfur-bearing gases such as H₂S react with silver solder or silver-plated components, forming conductive silver sulfide.
This causes short circuits across contacts, especially in low-voltage environments like data buses and memory modules.
Silver corrosion is particularly problematic in environments with high humidity and poor filtration, as it propagates quickly and often remains undetected until devices fail.
Hard Disk Crash Statistics
Contaminant buildup on hard disk platters is another failure mode. Even microscopic particulate intrusion can cause head crashes in sensitive spinning disks.
According to studies by major hardware manufacturers, facilities located in high-traffic or industrial zones have up to 3× higher hard drive failure rates due to airborne contaminants compared to filtered environments.
To reduce this risk, hard disk OEMs often recommend ISO Class 8 or better air quality, combined with temperature and humidity regulation.
How to Measure Corrosivity in Airborne Environments
Understanding and controlling airborne corrosivity is essential in protecting sensitive electronics, infrastructure, and critical environments. To evaluate the risk and severity of corrosion in a given space, several standardized methods and tools are used. These approaches help facilities assess long-term exposure and identify the need for filtration upgrades or environmental controls.
ISO 9223 Corrosion Coupons
The ISO 9223 standard provides a classification system for atmospheric corrosivity based on exposure testing using metal coupons.
In this method, small samples of metals like carbon steel, zinc, copper, or aluminum are exposed to the environment for a set period—typically 30, 90, or 365 days.
After exposure, the mass loss due to corrosion is measured and used to calculate corrosion rates in micrometers per year (µm/year). ISO 9223 categorizes environments into corrosivity classes (C1 to C5), ranging from very low (C1) to very high (C5).
For accurate interpretation:
Mass loss is used to quantify chemical attack on a general scale.
Thickness loss helps determine mechanical weakening or functional degradation.
The standard is widely used in infrastructure, telecommunications, and outdoor installations. For more on ISO 9223, refer to ISO.org.
Copper-Silver Reactivity Monitors
These are small, passive sensors used primarily in data centers, cleanrooms, and museums to detect corrosive gas concentrations. The monitor consists of polished copper and silver strips exposed to ambient air.
Over 30 days, the discoloration or tarnish rate is analyzed using colorimetric or microscopic methods.
These rates are then converted to equivalent ISA G1–G4 corrosivity classes, helping users assess how airborne gases like sulfur compounds or chlorides are affecting electronics.
This method is ideal for early detection of corrosive threats in environments with low ventilation or variable outdoor air intake.
Real-Time RH and ΔT Logging
Corrosivity is not determined by pollutants alone—relative humidity (RH) and temperature gradients (ΔT) play major roles.
High RH accelerates electrochemical reactions and promotes condensation on surfaces, especially when dew point is approached or exceeded.
Installing real-time sensors to monitor:
RH and temperature
Dew point proximity
Airflow changes or thermal shock
…helps predict corrosion-prone periods and optimize HVAC settings. Many Building Management Systems (BMS) now support continuous environmental logging, enabling trend analysis and maintenance planning.
Interpreting Mass Loss vs. Thickness Loss
Mass loss measures the total amount of material lost and is used to calculate corrosion rate over time.
Thickness loss is more relevant for mechanical or electrical performance, particularly where conductive pathways or structural tolerances are critical.
In electronics or precision environments, even minor thickness reductions can lead to connectivity loss or mechanical instability.

Prevention Toolbox for Corrosion Control in Air Environments
Preventing corrosion in critical environments requires a combination of protective materials, engineered filtration systems, and intelligent monitoring. The right mix of strategies can extend equipment life, preserve structural integrity, and reduce operating costs. Below are proven tools and techniques used across industries to combat airborne corrosion.
Protective Coatings
Applying anti-corrosive coatings to exposed metal surfaces is one of the most cost-effective ways to slow corrosion. These include:
Epoxy and polyurethane coatings: used on steel surfaces in industrial plants and HVAC components.
Zinc-rich primers: which act as sacrificial barriers to protect base metals.
Electrostatic powder coatings: often applied to electrical enclosures or server racks to protect from airborne pollutants and humidity.
Proper surface preparation and coating thickness are critical to ensuring long-term durability.
Hot-Dip Galvanizing
Hot-dip galvanizing involves immersing steel in molten zinc, creating a metallurgically bonded coating that offers both barrier and sacrificial protection. It is particularly effective for:
Outdoor structures exposed to salt spray or industrial air
HVAC ductwork and support systems
High-humidity or high-condensation zones
Galvanizing provides long-lasting defense in ISO C3–C5 corrosivity zones and can be paired with paint systems for duplex protection.
Cathodic Protection
Cathodic protection is a technique used to prevent corrosion in metallic structures by redirecting electrochemical currents. It is commonly applied in:
Underground pipelines
Reinforced concrete structures (bridges, parking garages)
Storage tanks and marine infrastructure
There are two main types:
Sacrificial anode systems
Impressed current systems
Though typically used for buried or submerged assets, similar concepts can be applied to shield air-exposed components in harsh environments.
Molecular Filtration
For airborne contaminants like SO₂, NO₂, H₂S, and ozone, molecular (gas-phase) filtration is the frontline solution. These systems work by adsorbing or chemically neutralizing gaseous pollutants.
Chemical scrubbers: use impregnated media to trap acid gases in high-volume airflow systems.
Molecular media (e.g., activated carbon, potassium permanganate): remove corrosive gases from ambient air.
Compact filters: integrate particulate and gas-phase layers to target both fine PM and corrosive vapors in space-limited applications.
These are vital in data centers, museums, cleanrooms, and coastal industrial facilities.
Smart Differential Pressure (DP) Sensors
Monitoring pressure drop across filters helps ensure timely maintenance and reliable airflow. Smart DP sensors offer:
Real-time tracking of filter loading
Alerts when filters approach end-of-life
Integration with Building Management Systems (BMS)
This not only prevents overuse and system strain but also ensures that protective measures (like gas-phase filters) remain effective.

ROI of Corrosion Control in Air-Handling Environments
Investing in corrosion control is not just about preventing damage—it delivers measurable returns through energy savings, extended equipment life, and reduced operational interruptions. When properly implemented, corrosion mitigation strategies often pay for themselves in a short period and continue delivering value over the life of the facility.
Energy Savings from Economizers and Clean Airflow
Air-side economizers are used in many HVAC systems to reduce energy consumption by bringing in cooler outdoor air. However, this process also introduces corrosive gases like SO₂, NO₂, and H₂S—especially in urban or industrial zones. Without proper molecular filtration, the cost of corrosion can outweigh the savings.
Adding gas-phase filters to economizer intake enables continued free cooling while protecting sensitive equipment. The result is a double benefit:
Lower HVAC energy use (reduced compressor load)
Protection of internal coils, electronics, and ductwork from acid gas attack
Case study data shows that in data centers or clean production environments, molecular filters can extend coil life by 5–7 years, saving thousands in replacements and unscheduled shutdowns.
Extended Asset Life and Deferred Capital Expense
Corrosion leads to premature failure of:
Server components
HVAC coils and fans
Power distribution boards
Structural supports and enclosures
By installing effective air filtration and environmental monitoring, many facilities extend asset life by 30–50%, delaying costly replacements. For example:
A $60,000 AHU coil replacement deferred by 5 years translates to $12,000/year in avoided depreciation.
A single high-end server board replacement costs ~$5,000. Corrosion prevention extends service intervals.
Avoided Downtime and Maintenance Costs
Unexpected equipment failures due to corrosion can cause:
Production stoppages
Emergency callouts
Data loss or system crashes
In electronics facilities or hospitals, even 1 hour of downtime can cost thousands—or pose safety risks. Corrosion protection helps:
Maintain uptime
Reduce emergency maintenance
Minimize warranty claims and customer disruptions
Payback Example: Filtration Investment in a Data Facility
Scenario:
$10,000 investment in dual-layer filtration (particulate + molecular)
Filters protect $500,000 in IT hardware
Avoided damage estimated at $50,000 over 3 years
Payback:
$50,000 savings ÷ $10,000 investment = 5x ROI in under 36 months
Plus, improved IAQ and less frequent HVAC servicing
Long-Term Value
Corrosion control often yields simple payback in 1–2 years, with continued benefits over the equipment’s lifespan. By proactively addressing airborne corrosion risk, facility managers can:
Reduce capital expenditure
Improve energy efficiency
Protect mission-critical operations
For data-driven insights into asset risk and corrosion cost modeling, see NACE International Reports and ASHRAE Technical Committee on Particulate and Gas Contaminants.

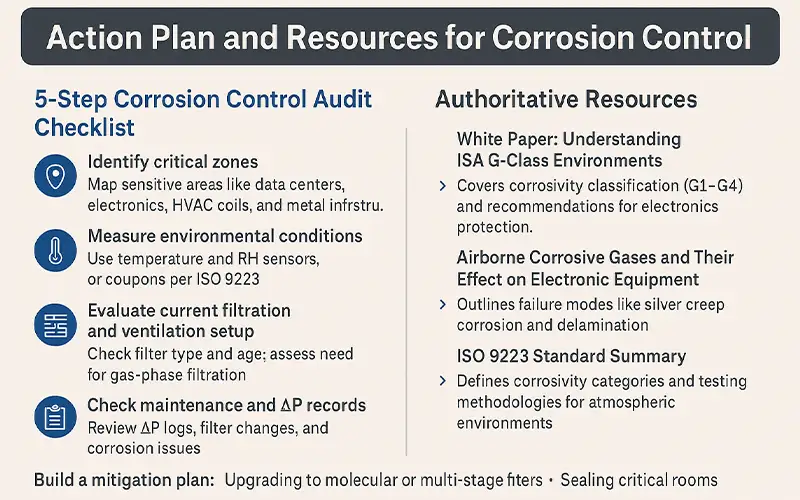
Action Plan and Resources for Corrosion Control
Implementing an effective corrosion control strategy requires a structured approach, starting with understanding your environment and system vulnerabilities. Below is a step-by-step action plan, supported by authoritative resources, to help you assess and manage airborne corrosion risks in your facility.
5-Step Corrosion Control Audit Checklist
Identify critical zones
Map out areas housing sensitive equipment, electronics, HVAC coils, and metal infrastructure. Pay special attention to data centers, control rooms, and humid or polluted intake locations.
Measure environmental conditions
Deploy temperature and relative humidity (RH) sensors. For a deeper diagnosis, use corrosion classification coupons per ISO 9223 or copper-silver reactivity monitors to quantify corrosive gas exposure over time.
Evaluate current filtration and ventilation setup
Review the type, efficiency, and age of air filters in use—especially outside air intakes and return air systems. Check if gas-phase filtration is present or needed based on contaminant profile.
Check maintenance and ΔP records
Look at historical pressure drop (ΔP) logs, filter replacement intervals, and signs of corrosion in ductwork or electronics. Corrosion is often visible near vents, connectors, or exposed terminals.
Build a mitigation plan
Based on risk level and system capability, develop a strategy that may include:
Upgrading to molecular or multi-stage filters
Sealing critical rooms
Introducing active air monitoring
Scheduling condition-based filter changeouts
Authoritative Resources
White Paper: Understanding ISA G-Class Environments
Covers corrosivity classification (G1–G4) and recommendations for electronics protection.
Read from AMPP (formerly NACE) →
Guide: Airborne Corrosive Gases and Their Effect on Electronic Equipment
Outlines failure modes like silver creep corrosion and delamination.
Available from ASHRAE TC 2.3 →
ISO 9223 Standard Summary
Defines corrosivity categories and testing methodologies for atmospheric environments.
View ISO guidance →
Ready to Get Started?
If you’re unsure how to interpret test results or select the right filtration media, consult an air quality specialist. Our team at Clean-Link can help you:
Recommend the right filter grade for your application
Perform site audits and corrosivity assessments
Provide tested, energy-efficient filter products for gas-phase and particulate control
Contact Clean-Link for Corrosion Control Support →
Final Thoughts
Airborne corrosion is a hidden threat that shortens equipment lifespan, compromises infrastructure, and causes costly downtime—often without visible warning. By taking a structured approach to environmental monitoring, filtration upgrades, and proactive maintenance, facility managers can protect critical assets and maintain long-term operational stability.
At Cleanlink, we offer a full suite of industrial air filtration solutions specifically engineered to combat corrosion risks. From multi-stage HVAC filters to molecular filtration systems targeting SO₂, NO₂, and H₂S, our products are trusted by data centers, museums, manufacturing facilities, and infrastructure operators worldwide.
Clean-Link Offers Quality Filter Solutions for HVAC Systems in Commercial Industry









